All posters will be exhibited during exhibit hall breaks. Due to COVID-19 traveling restrictions, some posters may not be available to be viewed in person.
The scientific committee will select the best scientific poster abstract based on ingenuity and content. The winning presenter will be announced during the Closing Reception on October 16th and will be awarded a prize of $300, sponsored by Medical Sciences, an international open access journal providing a platform for advances in basic, translational, and clinical research.
Click an abstract title below to view full abstract details.
Return to the Meeting Access Page
| Abstract 01: Quantification of Epicardial Fat Volume as a Novel Cardiovascular Risk Marker in Asymptomatic Subjects for Early Detection of Cardiovascular Disease M. El-Shahaway, MD, A. Sabatini, PE, MS, L. Izadi, BS, S. Tucker, BSN, F. Yturralde, MD Cardiovascular Ctr of Sarasota, FL |
| Abstract 02: Association of hyperuricemia with length of stay, hospital readmissions and in-hospital mortality in patients admitted with an acute heart failure exacerbation J. Leso, W. Itani, M. Harutyunyan, H. Patel, M. Torosoff Albany Medical Center |
| Abstract 03: linical and Echocardiographic Findings in Black Heart Failure Patients with Rheumatoid Arthritis. A. Hasan, S. Zaidi, J. Casillas Gonzalez, A. Tayadoni, H. Chandrakumar, M. Freilich, L. Zonnoor, A. Corominas, L. Salciccioli, I.M. McFarlane SUNY Downstate Health Sciences University |
| Abstract 04: Blood Pressure Improvement in People Using a Digital Health Solution for Comprehensive Diabetes Self-management A.Kumbara, MS, MBA, A.K. Iyer, PhD, MBA, M. Shomali, MD, CM Welldoc, Inc., Columbia, MD, U.S.A. |
| Abstract 05: Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Severe Hypertriglyceridemia - Initial Baseline Data from an Ongoing Phase 2 Study D.L. Bhatt, MD, MPH1, J.J.P. Kastelein, MD2, T. Parli, MD3, R.W. Charlton, MD3, C.L. Hartsfield, PhD3, S. Feng, PhD3, H. Mansbach, MD3 1. Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2. University of Amsterdam, Amsterdam, Netherlands; 3. 89bio, San Francisco, CA, USA |
| Abstract 06: BIO89-100 Demonstrated Robust Reductions in Liver Fat, Improved Metabolic Parameters, Favorable Tolerability and Potential for Weekly (QW) or Every 2 Weeks (Q2W) Dosing in a Phase 1b/2a Placebo-Controlled, Double-Blind, Multiple Ascending Dose Study in NASH. J.P. Frias, MD1, G. Ortiz-Lasanta, MD2, C.L. Hartsfield, PhD3, L. Tseng, PhD3, R.W. Charlton, MD3, H. Mansbach, MD3, M. Margalit, MD4, R. Loomba, MD5. 1. National Research Institute, Los Angeles, CA, USA; 2. FDI Clinical Research, San Juan, PR, USA; 3. 89bio, San Francisco, CA, USA; 4. 89bio, Herzliya, Israel; 5. NAFLD Research Center, University of California at San Diego, La Jolla, CA, USA. |
| Abstract 07: Cardiovascular risk factors in patients with coronary artery disease who are re-hospitalized in the Service of Cardiology G.L. Don, MD1, M.F. CenturiÓn2, E.Y. Macbeth3 1. Chief of the Service of Cardiology. San Martín Hospital, Entre Ríos, Argentina; 2. Epidemiology Residence Directorate of Epidemiology, Ministry of Health of the Province of Entre Ríos, Argentina; 3. Cardiology Residence. San Martín Hospital, Paraná, Entre Ríos, Argentina |
| Abstract 08: Assessment of Heart Failure risk and Characteristics in the Black population with Gout. H.P. Chandrakumar, A.V. Puskoor, S. Chillumuntala, D.C. Mora, T. Gupta, A. Tadayoni, S. Sharif, S.L. Zonnoor, L. Salciccioli, I.M. Mcfarlane SUNY Downstate Health Sciences University |
| Abstract 09: Burden of influenza and use of antiviral treatments in Cardiovascular Disease Patients M. Corral1, T. To1, R.de Cassia Castro1, S. Arndorfer2, S. Wang2, J. Stephens1 1. Genentech, Inc.; 2. Genesis Research |
| Abstract 10: The Impact of Age, Weight, and Gender on the Development of Superficial Venous Disease M. Paudel, M. Shah, P.S. Chopra, A. Chaturvedi, S. Singh, Y. Goyal, A. Kapoor MIMIT Health |
| Abstract 11: Real-world treatment patterns among patients with atherosclerotic cardiovascular disease (ASCVD) using lipid-lowering therapy in the HealthCore Integrated Research Database (HIRD) J.L. Smith1, L. Ndri2, Z. Jiang1, M. Singhal1, B. Electricwala1 1. HealthCore, Inc; 2. College of Population Health, Jefferson University |
| Abstract 12: Efficacy and Safety of Finerenone in Patients with CKD and T2D by Baseline Insulin Treatment P. Rossing, MD1,2, R. Agarwal, MD, MS3, S.D. Anker, MD4, G. Filippatos, MD5, B. Pitt, MD6, L.M. Ruilope, MD,7-9 R. MacIsaac, MD10, J. Wainstein, MD11,12, A. Joseph, MBBS,13 G.L. Bakris, MD14, on behalf of the FIDELIO-DKD Investigators. 1. Steno Diabetes Center Copenhagen, Gentofte, Denmark; 2. Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark; 3. Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN, USA; 4. Department of Cardiology (CVK), and Berlin Institute of Health Center for Regenerative Therapies, German Center for Cardiovascular Research Partner Site Berlin, Charité Universitätsmedizin, Berlin, Germany; 5. National and Kapodistrian University of Athens, School of Medicine, Department of Cardiology, Attikon University Hospital, Athens, Greece; 6. Department of Medicine, University of Michigan School of Medicine, Ann Arbor, MI, USA; 7. Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, Madrid, Spain; 8. CIBER-CV, Hospital Universitario 12 de Octubre, Madrid, Spain; 9. Faculty of Sport Sciences, European University of Madrid, Madrid, Spain; 10. Department of Endocrinology and Diabetes, St Vincent's Hospital Melbourne & University of Melbourne, Melbourne, Victoria, Australia; 11. Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; 12. Diabetes Unit, Edith Wolfson Medical Center, Holon, Israel; 13. Cardiology and Nephrology Clinical Development, Bayer AG, Berlin, Germany; 14. Department of Medicine, University of Chicago Medicine, Chicago, IL, USA. |
| Abstract 13: FIDELIO-DKD Study Analysis of Effects of Finerenone by Baseline A1C P. Rossing, MD1,2, E. Burgess, MD3, R. Agarwal MD MS4, S.D. Anker MD5, G. Filippatos MD6, B. Pitt MD7, L. M. Ruilope MD10, P. Gillard MD11, A. Joseph MBBS12, G.L. Bakris MD13 on behalf of the FIDELIO-DKD Investigators 1. Steno Diabetes Center Copenhagen, Gentofte, Denmark; 2. Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark; 3. Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 4. Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN, USA; 5. Department of Cardiology (CVK), and Berlin Institute of Health Center for Regenerative Therapies, German Centre for Cardiovascular Research Partner Site Berlin, Charité Universtätsmedizin, Berlin, Germany; 6. National and Kapodistrian University of Athens, School of Medicine, Department of Cardiology, Attikon University Hospital, Athens, Greece; 7. Department of Medicine, University of Michigan School of Medicine, Ann Arbor, MI, USA; 8. Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, Madrid, Spain; 9. CIBER-CV, Hospital Universitario 12 de Octubre, Madrid, Spain; 10. Faculty of Sport Sciences, European University of Madrid, Madrid, Spain; 11. Department Endocrinology, University Hospital Leuven - KUL, Leuven, Belgium ; 12. Cardiology and Nephrology Clinical Development, Bayer AG, Berlin, Germany; 13. Department of Medicine, University of Chicago Medicine, Chicago, IL, USA. |
| Abstract 14: The Role of Leptin and Inflammatory Related Biomarkers in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome R. Al Assil MD MHSc1, J.W. Younger PhD2 1. University of British Columbia; 2. University of Alabama |
Abstract 01: Quantification of Epicardial Fat Volume as a Novel Cardiovascular Risk Marker in Asymptomatic Subjects for Early Detection of Cardiovascular Disease Purpose: We aimed to establish normal values for epicardial fat volume (EFV) in asymptomatic women and men with zero coronary calcium score (CAC) in normal or low risk subjects for cardiovascular disease. Methods: 2,756 asymptomatic subjects, aged 20-79, were screened for CVD risk via ECVDRS and ACC/AHA risk scores; a total of 596 were asymptomatic and 37% underwent cardiac CT calcium score and EVF quantification. 69 out of the 220 subjects had a zero calcium score. These 69 were further categorized into 3 groups: 1: n= 20 ECVDRS<3, ACC/AHA risk <5%, CAC 0, 2: n=16 w/elevated EFV and no abdominal visceral obesity; 3: n=33 had elevated EFV and abdominal visceral obesity. A normal EFV value was established for both women and men Results: We identified a normal EFV for females of 69 cm3 +/- 20 and for men 68 cm3 +/- 15. Elevated EFV with or without abdominal visceral adiposity and zero CAC, was associated with vascular abnormalities and in particular an abnormal blood pressure response rise to post mild protocol exercise (PME), hence endothelial dysfunction and elevated CV risk. Conclusions: Elevated EFV is a novel and independent cardiovascular risk marker and early detection of an abnormal volume, i.e. >100 cm3 +/-20 in female or male, who had a zero CAC, may correlate with early vascular disease and mandate early aggressive lifestyle and diet modifications, complemented by early and aggressive medical therapy in order to delay or may prevent major cardiovascular events (MACE). |
Abstract 02: Association of hyperuricemia with length of stay, hospital readmissions and in-hospital mortality in patients admitted with an acute heart failure exacerbation Purpose: In acute congestive heart failure (CHF) management, uric acid levels increase with IV diuresis, but an acute therapeutic uric acid lowering was not found to decrease BNP levels or improve LV ejection fraction. Hyperuricemia (HU) has been noted associated with increased mortality in patients with chronic CHF. However, an association between HU and outcomes of acute CHF has not been well investigated. Methods: To study association between HU and length of stay (LOS), hospital readmission(s) and in-hospital mortality in acute heart failure, we conducted a single tertiary academic medical center retrospective cohort study of 586 consecutive patients with known uric acid level. Acute heart failure diagnosis was ascertained according to the GWG-HF definitions. Uric acid values were divided in quartiles as less than 3.39 (UAQ1), 4-6.79 (UAQ2), 6.8-10.19 (UAQ3) and 10.2 mg/dL or above (UAQ4). The normal reference range for our laboratory is 3.6-8.0 mg/dL in males and 2.6-6.8 mg/dL in females. Results: Of the study patients, 39% (226) were females, 68+/-15 years old, 40% (236) with history of diabetes, 70% (410) with treated hypertension, 47% (275) with history of CAD, and 37% (218) with history of chronic kidney disease (CKD), including those on hemodialysis. HU was not associated with increased prevalence of diabetes, hyperlipidemia, hypertension, or prior known CAD or CKD. Uric acid levels were significantly increased in patients who expired (12.3+/-3.3 vs. 7.6+/-2.9 in survivors, p=0.004). Furthermore, there were strong trends towards longer LOS (UAQ1 7.8+/-7.9 vs. UAQ2 8.6+/-10.9 vs UAQ3 9.5+/-10.5 vs. UAQ4 8.5+/-6.3 days, p=0.7) and more frequent readmissions associated with increasing levels of uric acid (UAQ1 32%, UAQ2 31%, UAQ3 38%, UAQ4 47%, P=0.06). Conclusions: In patients admitted with acute heart failure, elevated uric acid levels portend poor prognosis and are associated with increased mortality. Increased uric acid levels may also aid in identifying acute CHF patients who require increased hospital resource utilization by prolonging LOS and increasing re-admission rates. |
Abstract 03: Clinical and Echocardiographic Findings in Black Heart Failure Patients with Rheumatoid Arthritis. Purpose: Studies have reported higher incidence of heart failure among rheumatoid arthritis (RA) patients with heart failure with preserved ejection fraction being most prevalent in White RA cohorts. Previous work by our group demonstrated a prevalence of heart failure of 14.8% in the Black RA population. Utilizing clinical and echocardiographic data, we aimed to further characterize left ventricular function and associated risk factors. Our hypothesis was that HF with preserved EF (diastolic heart failure) will be the predominant type of HF among Black RA patients similarly as described for Caucasian RA patients with HF. Methods: Cross-sectional analysis of electronic medical record data of RA patients with HF was compared to age, sex, and race matched cohort of RA patients without HF. 2D-echocardiograms of RA-HF were reviewed for left ventricular ejection fraction, wall motion abnormalities and Global longitudinal strain/Relative wall thickness calculations. Descriptive statistics using SPSS version 29 were applied; logistic regression model was used to assess the strength of association between HF and cardiovascular risk factors. Results: Sixty-four patients with RA-HF were studied, mean age 69.6±1.38 (± SEM); 87.3% were Black, 84.4% were women, mean BMI was 29.6±1.07 Kg/m2. Compared to the RA without HF cohort, RA-HF patients had significantly higher rates of HTN, CKD and atrial fibrillation, 66.7% had ≥3 CV risks. 2D-echos demonstrated LVEF <50% in 37.7%, and LVEF 50% in 62.3%. Diastolic dysfunction (DD) was found in 37% with grade 1 DD being most prevalent (75%). Wall motion abnormalities was found in 43.1% of the patients. Global Longitudinal Strain was -8.41± 1.85, left ventricular mass was 187.03 ± 8.66 and Relative Wall Thickness was 0.399 ± 0.001. RA patients with HTN had an OR of 4.7 (1.5-14.53 CI) for HF, (95% CI), p< 0.01 and those who had smoking history had 3.5 times the risk of developing HF (OR of 3.5) (1.091-11.7CI) (95% CI), p< 0.01. Conclusions: Heart failure with preserved ejection fraction was the most prevalent among the Black RA patients with HF which is similar to the HF findings observed in Caucasian RA patients. Black RA patients with HF had higher rates of HTN than RA patients without HF. HTN and smoking conferred the highest odds of HF in this patient population. The low Global Longitudinal Strain found in the study patients predicts higher morbidity and mortality; further studies are needed to confirm our findings in Black RA patients with HF.
|
Abstract 04: Blood Pressure Improvement in People Using a Digital Health Solution for Comprehensive Diabetes Self-management Purpose: There is a growing recognition of the need to develop digital health solutions that address the total health of a user, including management of significant comorbidities. Hypertension affects roughly 70% of people with diabetes (PWD) and is twice as common in PWD compared to those without. Moreover, hypertension in PWD amplifies the risk of chronic kidney disease, cardiovascular complications, ischemic cerebrovascular disease, retinopathy, and sexual dysfunction (1). We have previously shown in multiple studies that a mobile application that supports diabetes self-management and delivers clinical decision support to healthcare providers can lower A1C by approximately 2.0% (2,3). Though this digital diabetes solution focuses on glucose control, medication management, and nutrition, the purpose of the current study is to examine user engagement with the app with regard to the important comorbidity of hypertension. In addition, since the current version of the app also provides some clinical coaching on hypertension and delivers decision support to clinicians around hypertension, we wanted to explore blood pressure outcomes. Methods: Data from 84 users of the digital solution were analyzed. Our primary outcome variable was a meaningful improvement in blood pressure (BP) from baseline to month 3 of using the digital health solution. Meaningful improvement in BP was defined as at least a 5 mmHg drop in systolic BP from baseline to month 3. To identify important self-management behaviors that correlate to our meaningful BP drop outcome variable, we included the first 3 months of engagement data and user demographics as predictor variables in a logistic regression model. We also statistically validated BP improvement among the 84 users split into three groups; Group 1 included users with average baseline systolic BP greater than 140 mmHg (n=12), Group 2 included users with average baseline systolic BP greater than 130 mmHg (n=34), and Group 3 included users with average baseline systolic BP less than or equal to 130 mmHg (Group 3, n=50). We performed a paired, one-tailed t-test to test our null hypothesis of no significant change in average BP from baseline to month 3 for each group. Results: Results of a logistic regression indicated that, logging exercise, sleep, along with BP annotations were significant predictors (p<0.05) of a meaningful improvement in systolic BP. Group 1 on average had a 12 mmHg drop in BP from baseline to month 3 (p = 0.0046). Group 2 on average had a 7.2 mmHg drop in BP from baseline to month 3 (p=0.0004), and Group 3 had no significant change in BP from baseline to month 3. Conclusions: Although entering BP was not required in the digital solution, we found that users of a diabetes coaching app had significant engagement with logging BP in the product. Meaningful improvements in BP were more likely to occur in users who also tracked other activities such as exercise and sleep. Significant reductions in BP were identified in those users whose baseline systolic BP was elevated. In summary, based on this sample of users, digital health solutions for people with diabetes that support blood pressure self-management have the potential to help diabetes patients with the common and serious comorbidity of hypertension. Further studies with larger number of users may help us to uncover the mechanisms for improving BP such as medication adherence, improved activity or sleep, or dietary modifications. 1. Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007 Oct;3(10):667. 2. Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011 Sep;34(9):1934-42. 3. Shearer D, Iyer A, Peeples M. A Payer Digital Health Study Shows Scalable Approach to Cost Savings and Outcomes. J Diabetes Sci Technol. 2021 Mar;15(2):521-522. |
Abstract 05: Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Severe Hypertriglyceridemia - Initial Baseline Data from an Ongoing Phase 2 Study
Methods: Descriptive statistics summarize baseline characteristics of screened/enrolled SHTG participants opting to obtain MRI-PDFF. Results: Demographic characteristics were as follows: age range 40-70 years, male 54.5%, white 100%, Hispanic/Latino 36.4%, BMI range 27.4 - 40.1, T2D 27.3%, and on background lipid therapy 36.4%. Metabolic baseline measurements included TG median 615 mg/dL (range 499-1054), glucose median 119 mg/dL (range 82-254), HbA1c median 5.6% (range 5.2-9.8), HOMA-IR median 7.98 mg/dL x mlU/L (range 3.08 - 26.45) and Adipo-IR median 16.10 mmol/L x U/mL (range 6.87 - 33.65). Baseline MRI-PDFF demonstrated clinically meaningful liver fat content (MRI-PDFF 5%) in 100% of these first 11 participants (range 6.2% -29.0%). The percentage of hepatic steatosis did not appear to correlate with baseline TG values, BMI, or T2D status. Presented results will be updated for any new participants with baseline MRI-PDFF data. Conclusions: The prevalence of hepatic steatosis was greater than expected in this study population, especially given that not all the patients were obese, and the majority did not have T2D. Patients with SHTG have an increased risk for multiple serious conditions such as cardiovascular disease and acute pancreatitis, however these data suggest they also have substantial risk of developing NAFLD. Given the potential broad metabolic benefits of BIO89-100, including improvements in liver fat, circulating TGs, and glycemic control, it is important to understand the prevalence of NAFLD in patients with SHTG. The preliminary baseline findings from ENTRIGUE suggest that routine assessment of hepatic steatosis may be warranted in SHTG patients. |
Abstract 06: BIO89-100 Demonstrated Robust Reductions in Liver Fat, Improved Metabolic Parameters, Favorable Tolerability and Potential for Weekly (QW) or Every 2 Weeks (Q2W) Dosing in a Phase 1b/2a Placebo-Controlled, Double-Blind, Multiple Ascending Dose Study in NASH. Purpose: It is well established that nonalcoholic steatohepatitis (NASH), in addition to the hallmarks of hepatic steatosis, inflammation and ballooning with and without fibrosis, is often associated with systemic alterations such as aberrant lipid profile, insulin resistance and co-morbidities including obesity, metabolic syndrome and type 2 diabetes (T2D). Indeed, cardiovascular disease is the leading cause of morbidity and mortality in this population. Therefore, an ideal treatment for NASH should simultaneously address the liver manifestations of the disease while targeting the underlying metabolic overload driving the hepatic pathology. Fibroblast growth factor 21 (FGF21) is an endogenous metabolic hormone that regulates carbohydrate, lipid and energy metabolism. FGF21 analogs have demonstrated capability to improve both hepatic and metabolic abnormalities in NASH. BIO89-100 is a long-acting glycoPEGylated FGF21 analog, with promising tolerability and pharmacodynamic effects and is the only FGF21 analog with the potential for once every 2 weeks (Q2W) dosing. Methods: This Phase 1b/2a trial enrolled 81 subjects with liver fat 10% by MRI-PDFF and either biopsy-confirmed NASH (BC-NASH) or phenotypic NASH (PNASH: central obesity with either T2D or with evidence of liver injury by ALT or FibroScan vibration controlled transient elastography score above defined thresholds). Subjects were randomized to 12 weeks of treatment at one of 6 doses (3, 9, 18 or 27mg weekly [QW]; 18 or 36mg Q2W) or placebo. Key endpoints were safety, tolerability, pharmacokinetics, change in liver fat content as measured by MRI-PDFF and liver and metabolic markers. Results: Baseline characteristics were similar between pooled BIO89-100 vs. pooled placebo groups, and between BC-NASH vs. PNASH subjects. At week 13, all BIO89-100 dose groups showed significant relative reductions up to 70% in MRI-PDFF (placebo adjusted p<0.001). Up to 88% of BIO89-100 subjects achieved 30% MRI-PDFF reduction vs. baseline (p<0.001), while up to 71% of subjects achieved the higher threshold of 50% relative fat reduction in the absence of weight loss (p<0.0004). Decreased liver fat was accompanied by a corresponding decrease in liver fat volume (LFV) of up to 305 mL or 65% from baseline (p<0.001). Significant decreases in ALT vs. placebo were observed with BIO9-100, maximal with 27 mg QW (30 U/L decrease from baseline, p<0.001) with the most prominent reduction in the subgroup (n=17) with baseline ALT>45 U/L (35 U/L decrease from baseline, p<0.05). Additionally, reductions of up to 28% were observed in levels of PRO-C3, an emerging non-invasive biomarker of fibrosis. Metabolic benefits of BIO89-100 included a favorable effect on lipids with significant improvements in triglycerides (TG; up to 28% reduction in overall population and up to 49% in the subgroup [n=15] with baseline TG 200 mg/mL); non-HDL cholesterol and LDL-C (reductions up to 15% and 16% respectively). Increases of up to 20% in HDL cholesterol were observed, as well as increases in adiponectin of up to 61%. There were no deaths or related serious adverse events; one BIO89-100 treated subject discontinued due to a treatment-related adverse event (localized skin rash). Mild increased appetite (15.9% in pooled BIO89-100) was the most common treatment-related AE. The frequency of gastrointestinal (GI) AEs compared favorably to placebo; diarrhea (BIO89-100 12.7%, placebo 22.2%) and nausea (BIO89-100 7.9%, placebo 16.7%) were the only GI AEs in 5% BIO89-100-treated subjects. There were no hypersensitivity reactions or adverse effects on blood pressure or heart rate. Conclusions: In this study, treatment with BIO89 100 for 12 weeks resulted in robust, clinically meaningful improvements in liver fat and volume and markers of liver stress and fibrosis. Additionally metabolic improvements as exhibited by reductions in triglycerides and LDL cholesterol and increases in HDL cholesterol and adiponectin were observed. These beneficial hepatic and systemic effects were shown in subjects who were treated by BIO89-100 QW or Q2W. Moreover, treatment was associated with a favorable safety and tolerability profile. There are currently no approved therapies available for NASH. While there are many therapeutic targets under investigation to date the results have been modest at best and many agents, while showing benefit on liver-related parameters, may in fact worsen lipids or result in weight gain that could potentially exacerbate cardiovascular risk. The data presented here indicate that BIO89-100 has significant potential as a new therapeutic for the treatment of NASH to improve liver-related parameters while simultaneously shifting whole-body metabolism towards a more normal state in these patients. Collectively, these data support the further investigation of BIO89-100 in NASH and other metabolic diseases. A Phase 2b study in NASH is currently underway, as well as an ongoing proof of concept study in patients with severe hypertriglyceridemia. |
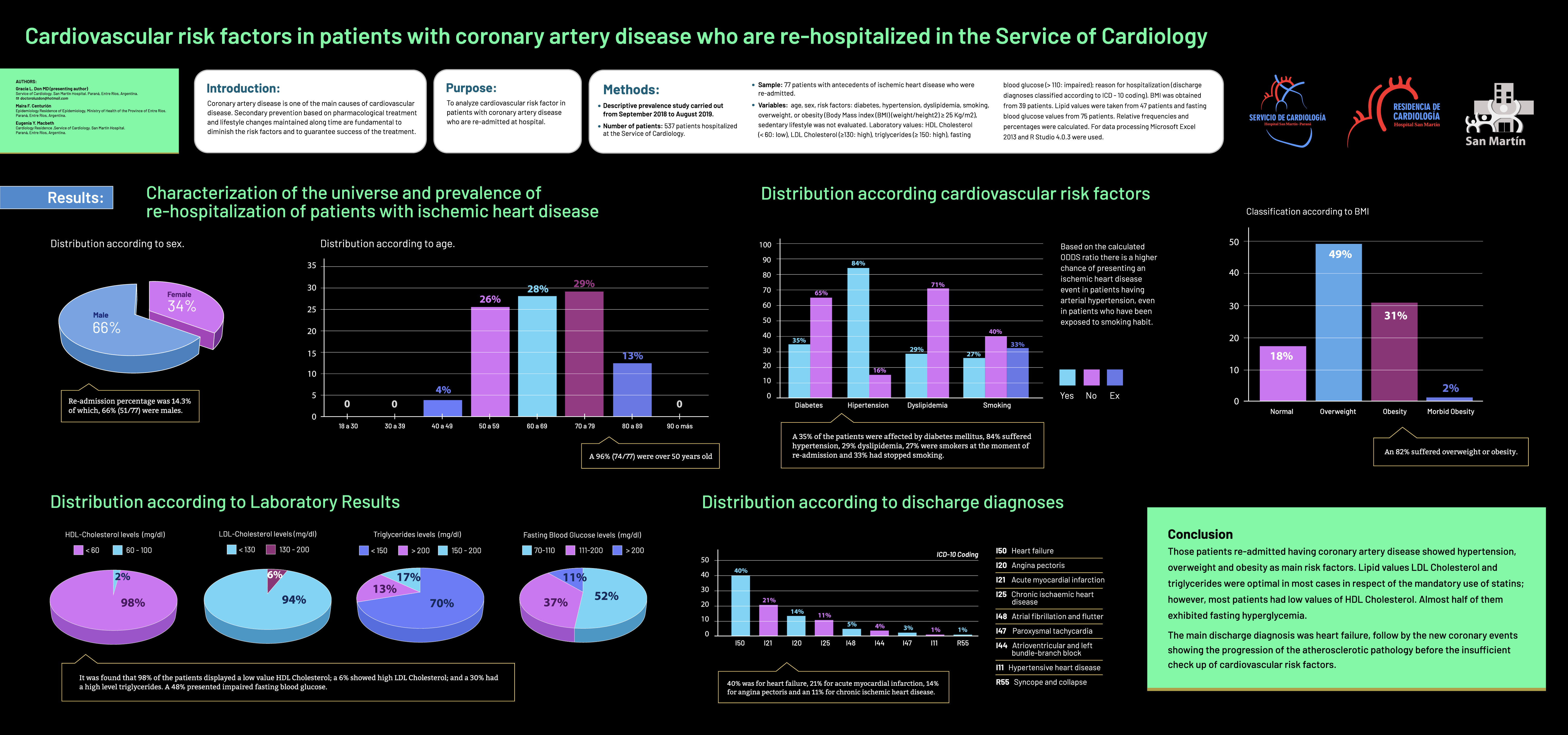
Abstract 07: Cardiovascular risk factors in patients with coronary artery disease who are re-hospitalized in the Service of Cardiology Purpose: Coronary artery disease is one of the main causes of cardiovascular disease. Secondary prevention based on pharmacological treatment and lifestyle changes maintained along time are fundamental to diminish the risk factors and to guarantee success of the treatment. Our objective was to analyze cardiovascular risk factors in patients with coronary artery disease who were re-admitted at hospital. Methods: Descriptive prevalence study carried out from September 2018 to August 2019. Number of patients: 537 patients hospitalized at the Service of Cardiology. Sample: 77 patients with antecedents of ischemic heart disease who were re-admitted. Variables: age, sex, risk factors: diabetes, hypertension, dyslipidemia, smoking, overweight, or obesity (Body Mass index (BMI) (weight/height2) (25 Kg/m2), sedentary lifestyle was not evaluated. Laboratory values: HDL Cholesterol (60: low), LDL Cholesterol (130: high), triglycerides (150: high), fasting blood glucose (110: impaired); reason for hospitalization (discharge diagnoses classified according to ICD - 10 coding). BMI was obtained from 39 patients. Lipid values were taken from 47 patients and fasting blood glucose values from 75 patients. We calculated relative frequencies and percentages. We used Microsoft Excel 2013 and R Studio 4.0.3 for data processing. Results: Re-admission percentage was 14.3% of which, 66% (51/77) were males and a 96% (74/77) were over 50 years old. As for risk factors in regards to the risk of cardiovascular disease: a 35% of the patients presented diabetes mellitus, 84% presented hypertension, 29% dyslipidemia, 27% were smokers at the moment of re-admission and 33% had stopped smoking. An 82% presented overweight, or obesity. As for the distribution of laboratory results: it was found that 98% presented a low value HDL Cholesterol; a 30% had a high level of triglycerides; and high LDL Cholesterol in a 6% of the patients. A 48% presented impaired fasting blood glucose. In regard to the discharge diagnoses: 40% was for heart failure, 21% for acute myocardial infarction, 14% for angina pectoris and an 11% for chronic ischemic heart disease. Conclusions: Those patients re-admitted having coronary artery disease presented: hypertension, overweight and obesity as main risk factors. Lipid values of LDL Cholesterol and triglycerides were optimal in most cases in respect of the mandatory use of statins; however, they presented low values of HDL Cholesterol. Almost half of them presented fasting hyperglycemia. The main discharge diagnoses was heart failure, favored by the predominant risk factors, though the prevalence of ischemic manifestations in different presentations shows the progression of the atherosclerotic pathology before the insufficient global checkup of cardiovascular risk factors. It is necessary to set up integral monitoring and educational programs, as well as constant follow ups of patients with coronary artery disease, which may lead towards active participation and access to cardiac rehabilitation in order to achieve treatment success and adherence.
|
Abstract 08 Assessment of Heart Failure risk and Characteristics in the Black population with Gout. Purpose: Prior studies have documented a higher prevalence of heart failure (HF) in the non-black gout population. Literature has shown a systolic dysfunction of about 12.7% among the Framingham Offspring cohort (Krishnan E, BMJ Open 2012;2: e000282). Using clinical and echocardiographic data, we aimed to compare the characteristics of left ventricular dysfunction and associated risk factors of HF in Black patients with and without gout. Methods: Cross sectional analysis of electronic medical record data of gout patients was compared to age, sex, and race matched cohort of the non-gout patients. Clinical parameters and 2D echocardiograms were reviewed for the patients with gout and heart failure. Left ventricular ejection fraction (LVEF), left ventricular wall motion and size abnormalities, diastolic dysfunction was evaluated. HF patients with gout were further categorized based on LVEF % into three groups: <40%, 41-55% and >55%. Descriptive statistics using SPSS version 29 was applied; logistic regression model was used to assess the strength of association between HF and cardiovascular risk factors. Results: The gout population of 471 patients with a mean age of 63.7±0.53 years, 89% being Black, 63% men were compared to an age, sex, race matched non gout cohort. Body mass index (BMI) for those with gout was 31.3±0.35 kg/m2 compared to 28.2± 0.2 kg/m2 in those without gout (p<0.01). The gout patients had higher rates of Hypertension (HTN), Hyperlipidemia (HLD), Diabetes mellitus (DM), chronic kidney disease compared to non-gout cohort. Gout patients had a higher prevalence of heart failure with 45.2%(n=213) compared to controls with 9.4%(n=44). HF patients with gout were categorized utilizing the LVEF into 3 groups. Systolic dysfunction was encountered at a higher proportion with 46.3% in EF<40%, 22.7% in EF 41-55% and 31% in EF>55%. Diastolic dysfunction (DD) was found in 45.9% of which Grade 1 DD was observed in 70%. Left ventricular hypertrophy was found in 22.6% in gout patients compared to 24.2% in non-gout group (p = NS). The mean highest serum uric acid levels among the 3 gout subgroups were 10±0.60 mg/dL without an intergroup significance(p=NS). In the logistic regression model, the unadjusted Odds ratio (OR) of HF in patients with gout was 7.97 (5.5-11.4, 95% CI), p < 0.01. After adjusting for traditional risk factors including age, BMI, HTN, DM, HLD, the OR of HF in patients with gout was 7.1 (4.7-10.6, 95% CI), p< 0.01. There were at least one or more all cause readmissions in 54.1% of gout patients as opposed to only 19% in the non-gout group (p<0.01). Conclusions: Black population with gout had a very high prevalence of cardiovascular risk factors and congestive heart failure as well as hospital readmission compared to non-gout cohort. Systolic dysfunction (46.3%) was found to be more prevalent than previously studied in the Caucasian population (12.7%). Larger studies are needed to confirm our findings and develop management strategies in black gout patients with HF. |
Abstract 09: Burden of influenza and use of antiviral treatments in Cardiovascular Disease Patients Purpose: People with Cardiovascular Disease (CVD) are at higher risk for developing serious complications from flu. Among adults hospitalized with flu during recent flu seasons, heart disease was one of the most commonly-occurring chronic conditions about half of adults hospitalized with flu that season had heart disease. Studies have shown that flu illness is associated with an increase of heart attacks and stroke. These patients are also more susceptible to respiratory-related influenza complications. This study explored the effect of antiviral medication on influenza-related complications and healthcare resource utilization in CVD patients who contracted the flu. Methods: A retrospective claims analysis, using IBM Watson MarketScan Commercial claims data, in patients with cardiovascular disease by flu season (October to April of following year). The study period encompasses three flu seasons: 2016-2017, 2017-2018, 2018-2019. Patients were included if they had: 1+ inpatient diagnosis or 2+ outpatient diagnosis of the same CVD condition at least 30 days apart; Flu index date was the first flu diagnosis date within each season and included only patients with flu diagnosis as the primary diagnosis. Patients were 18 or older with continuous enrollment: 12 months pre-flu index (baseline) and 1-month post-flu index. Patients were excluded who received prophylaxis antiviral treatment, defined as any flu treatment in the month prior to flu index date. Cases (treated): Patients who received antiviral flu treatment within 2 days of index flu diagnosis. Excluded patients who received prophylactic antiviral flu treatment with 10-day supply as their index treatment. Controls (untreated): Patients who did not receive any antiviral flu treatment in the 30 days post index of flu diagnosis; Exclude all patients who were hospitalized between index flu and two days post-index. Treated and untreated CVD-flu patients were propensity score matched 1:1 (case:control) with nearest neighbor matching (without replacement). Results: There were n=4,323 treated and n=4,323 matched, untreated patients. Baseline characteristics were similar between the two groups; mean age and proportion of males were well balanced between the cohorts. Mean age at flu index date for treated and untreated, respectively was 63 vs 64 years; proportion of males was 51.4% vs 51.0%. Across treated and untreated cohorts, the majority of patients were located in the southern United States, covered under PPO/EPO insurance, 2018 had the highest ratio of flu index. Additionally, across all patients, many had a previous diagnosis of myocardial infarction, heart failure, atrial fibrillation and stroke. Mean Charlson Comorbidity Index (CCI) score was 1.41 for Treated and 1.45 for Untreated patients. After matching, in both Treated and Untreated cohorts: (n=4321) after 30 days; (n=4109) within 60 days; (n=3964) after 90 days and (n=3664) after 180 days. All cause hospitalizations: (At least 1 ER visit (30 days: 11.9% vs 15.8%, p<0.01), (60 days: 17.2% vs 22.0%, p<0.01), (90 days: 21.4% vs 25.9%, p<0.01), (180 days: 32.1% vs 34.6%, p=0.021); At least 1 pharmacy fill (30 days: 87.6% vs 78.9% p<0.01), (60 days: 95.0% vs 87.7% p<0.01), (90 days: 97.4% vs 90.6% p<0.01), (180 days: 98.8% vs 92.7% p<0.01); Any Respiratory-related HRU (30 days: 25.6% vs 33.1%, p<0.01), (60 days: 30.6% vs 37.8%, p<0.01), (90 days: 34.5% vs 41.4%, p<0.01), (180 days: 42.5% vs 47.9%, p<0.01); At least 1 outpatient visit (30 days: 25.3% vs 32.9%, p<0.01), (60 days: 30.4% vs 37.6%, p<0.01), (90 days: 34.3% vs 41.3%, p<0.01), (180 days: 42.4% vs 47.8%, p<0.01); At least 1 ER visit (30 days: 4.2% vs 5.7%, p<0.01), (60 days: 5.0% vs 6.9%, p<0.01), (90 days: 5.4% vs 7.5%, p<0.01), (180 days: 6.6% vs 9.1%, p<0.01); Specific to Cardiovascular-related outcomes (30 days: 8.5% vs 12.4%, p<0.01), (60 days: 13.7% vs 17.4%, p<0.01), (90 days: 17.6% vs 21.4%, p<0.01), (180 days: 25.9% vs 29.0%, p<0.01); Heart failure (30 days: 3.7% vs 6.7%, p<0.01), (60 days: 5.6% vs 8.5%, p<0.01), (90 days: 7.3% vs 10.1%, p<0.01), (180 days: 10.5% vs 12.6%, p<0.01); Acute kidney failure (30 days: 0.7% vs 1.7%, p<0.01), (60 days: 0.9% vs 2.0%, p<0.01), (90 days: 1.0% vs 2.2%, p<0.01), (180 days: 1.7% vs 2.8%, p<0.01) were the most statistically significantly different. Conclusions: Among adults with CVD hospitalized with flu during three recent flu seasons, heart disease was one of the most commonly occurring chronic conditions. Cardiovascular patients who contracted the flu and took antiviral medication suffered fewer all-cause hospitalizations, respiratory-related influenza complications, underlying disease complications and overall healthcare resource utilization compared to CVD patients who were not prescribed antiviral treatments. These conditions worsened over time. |
Abstract 10: The Impact of Age, Weight, and Gender on the Development of Superficial Venous Disease Purpose: The purpose of this investigation was to identify risk factors for Superficial Venous Disease (SVD) among MIMIT Health's patient population. Through analyzing these determinants of health, MIMIT Health can efficiently recognize and diagnose SVD in order to improve the quality of life for our patients. Patient care can also be standardized and optimized with a greater understanding of common experiences amongst our patient population. Methods: A comprehensive analysis was conducted on 268 patients who were diagnosed with SVD and received one or more minimally invasive treatment procedures from our institution. Three separate analyses were made to compare procedure codes to age, gender, and weight. Age was considered for patients 20 years and older, gender was classified as either male or female, and weight was grouped by standardized BMI categories. Each patient was given conservative treatment of compression stockings for a minimum of six weeks before considering sclerotherapy. Our most common procedures were injection of Varithena and Endovenous Laser Treatment (EVLT). The former involved a guided injection of a non-compounded foam sclerosant with ultrasound compression maneuvers. The EVLT procedure involves using laser heat to scar incompetent superficial veins. 1170 procedures associated with 6 CPT codes regarding these two treatment types were analyzed for cost and outcome for these 268 patients. Results: Our patient population consisted of 178 females, 89 males, and 1 unknown. Out of the 268 patients, 27 were overweight, 17 were moderately overweight, 13 were morbidly obese, 13 were severely obese, 11 were normal, and 1 was severely underweight. The remaining patient populations' weights were not accounted for. 81.7% of this patient population was over the age of 50. Procedures with the CPT code of 36465 costed 2.5 million, 36478 required .8 million, 36475 required .3 million, and 36471 required .1 million. Conclusions: Conclusions made from analyzing this patient population are consistent with previous studies correlating these determinants of health to SVD. Female patients were more likely to require interventional treatment, most likely due to dynamic changes of the vascular system within women when pregnant. In addition, patients who were documented for BMI were primarily classified as overweight. An increase in weight has also been documented to be a risk factor for SVD due to excess pressure that weight puts on venous valves. In addition, increased weight is typically correlated to less activity levels which is harmful for these valves. In regards to age, our findings were also consistent with published literature. Older populations were more susceptible to needing treatments due to the severity of their symptoms. This thorough investigation of risk factors for SVD within our patient population aids in streamlining diagnoses and efficiencies of treatment at MIMIT Health.
|
Abstract 11: Real-world treatment patterns among patients with atherosclerotic cardiovascular disease (ASCVD) using lipid-lowering therapy in the HealthCore Integrated Research Database (HIRD) Purpose: Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in the US [1] and includes coronary heart diseases such as myocardial infarction, ischemic stroke, and peripheral artery disease. High levels of low-density lipoprotein cholesterol (LDL-C) is one of the primary causes of ASCVD. [2,3] The American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines recommend lipid-lowering therapies (LLTs) to reduce LDL-C by 50% and recommend that patients should be treated to an LDL-C <70 mg/dL.[2] Statins are the preferred first-line LLT, however around 80% of patients on statins do not achieve recommended LDL-C goals.[4] Other treatments, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitor monoclonal antibodies (PCSK9is), are recommended as next line of treatment. With these newer treatments, the knowledge regarding adherence, persistence, and discontinuation among LLTs remain limited. This study examined real-world treatment patterns of LLTs including statins, ezetimibe, and PCSK9is as well as LDL-C goal attainment among patients with ASCVD using a large claims-based database. Treatment patterns included: adherence, persistence, and discontinuation for LLTs, measured over 12 and 24 months from the first LLT claim after the first outpatient ASCVD visit during the predefined study period. Methods: The research questions were addressed using a retrospective, observational study using claims, eligibility, and outpatient lab data from the HealthCore Integrated Research Database (HIRD®) between October 1, 2014 - December 31, 2020 (study period). Patients 18 years with an ASCVD diagnosis between October 1, 2015 - December 31, 2019, were included. Index date was the earliest claim date for an LLT (statins, ezetimibe, PCSK9is) between first ASCVD diagnosis date and first ASCVD diagnosis date + 365 days/ 730 days for patients with 12/ 24-months of follow-up during study period. LLTs provided within 30 days of index date were considered as combination treatments for cohort assignments. Patients with 12 or 24 months continuous health plan enrollment after the index date (i.e., follow-up) were included. A subset of patients with 2 outpatient lab results during baseline (index date ± 90 days) and follow-up periods (index date + 183/365 to index + 365/ 730) days was used to report LDL-C change.' The outcomes of interest included adherence, persistence, discontinuation of LLTs, and LDL-C change. Adherence was defined as having proportion of days covered (PDC) 80%. PDC was calculated as days covered by a drug (or overlap days for combinations) during the follow-up period. Persistence was defined as the number of days from index until LLT discontinuation (<60 days allowed gap) or end of follow-up. Discontinuation was defined as having a gap in LLT days supplied ≥60 days without additional claims for the same LLT over the remainder of follow-up period. Results: Among ASCVD patients with 12 and 24 months of follow-up (n=642,005, n=461,050; respectively), only 65.1% and 68.1% patients used an LLT, respectively. Treated patient characteristics were similar across cohorts: the mean (SD) age was 66.5 (11.9) years, 63.8% male, and 19.1% had Medicare Advantage coverage. Conclusions: It was observed that 27.7-32.0% patients did not use any LLT after ASCVD diagnosis. The majority of ASCVD patients were treated with statin monotherapy. Adherence and persistence were similar across drug classes but declined over a 24-month period across all LLTs. Persistence across drug classes was found to be longer among monotherapies than combination therapies. About a third of ezetimibe and a quarter of PCSK9i users discontinued treatment during 24-months of follow-up. |
Abstract 12: Efficacy and Safety of Finerenone in Patients with CKD and T2D by Baseline Insulin Treatment Purpose: Finerenone is a novel, selective, nonsteroidal mineralocorticoid receptor antagonist that significantly reduced the risk of kidney and CV outcomes in patients with CKD and T2D in the FIDELIO-DKD trial, with no effect on blood glucose. In advanced T2D, insulin is often used to control glycemia. This analysis will report outcomes from the FIDELIO-DKD trial by baseline insulin treatment. Methods: In FIDELIO-DKD (NCT02540993), 5734 patients with T2D, UACR 305000 mg/g, eGFR 25<75 mL/min/1.73 m2 and treated with optimized RAS blockade were randomized to oral finerenone or placebo. The primary outcome was a composite of kidney failure, a sustained 40% eGFR decline from baseline, or renal death. The key secondary outcome was a composite of CV death, non-fatal MI, non-fatal stroke, or hospitalization for heart failure. Results: Of the 5674 patients analyzed, 3637 (64.1%) were treated with insulin or insulin analogues at baseline. Patients treated with insulin at baseline had a higher A1C and a longer duration of diabetes, with higher proportions of statin and GLP-1RA use, and lower use of other anti-hyperglycemic agents than those who were not. Finerenone did not affect A1C during the trial. The primary and key secondary CV outcome occurred in fewer patients treated with finerenone, with no between-group interaction (primary outcome: HR 0.85, 95% CI 0.73 0.98 with insulin; HR 0.79, 95% CI 0.64 0.96 without insulin; P-value for interaction 0.56; CV outcome: HR 0.82, 95% CI 0.69 0.97 with insulin; HR 0.95, 95% CI 0.74 1.23 without insulin; P-value for interaction 0.33). Hyperkalemia events were similar between groups (mean treatment difference between finerenone and placebo for treatment-emergent serum potassium >5.5 mmol/L, 11.1% with insulin and 13.4% without insulin), with a low incidence of hyperkalemia-related treatment discontinuation. Conclusions: Finerenone has a beneficial effect on kidney and CV outcomes in patients with CKD and T2D, irrespective of insulin use at baseline. |
Abstract 13: FIDELIO-DKD Study Analysis of Effects of Finerenone by Baseline A1C Purpose: Patients with CKD and T2D are at high risk of morbidity and mortality despite the use of guideline-directed therapies. Finerenone, a novel, selective, nonsteroidal mineralocorticoid receptor antagonist, reduced the risk of adverse kidney and CV outcomes in patients with CKD and T2D; here we report outcomes by baseline A1C. Methods: FIDELIO-DKD (NCT02540993) randomized 5734 patients from 48 countries to receive oral finerenone or placebo. Eligible patients had T2D, with a UACR of 30 5000 mg/g, eGFR<75 mL/min/1.73 m2 and received optimized RAS blockade. Patients with A1C >12% at screening were excluded. The primary outcome was a composite of kidney failure, sustained 40% decrease in eGFR from baseline, or renal death. The key secondary outcome was a composite of CV death, non-fatal MI, non-fatal stroke, or hospitalization for heart failure. Results: In the 5674 patients analyzed, the mean baseline A1C was 7.7% and mean diabetes duration was 16.6 years; finerenone did not affect A1C during the trial. Overall, 2948 patients had A1C = 7.5% and 2715 patients had A1C >7.5% at baseline. Patients with higher A1C levels were more likely to use insulin and had a longer duration of diabetes. After a median follow-up of 2.6 years, the primary outcome occurred in fewer patients treated with finerenone compared with placebo, this was observed in both the A1C 7.5% and >7.5% groups (18.7% vs 21.5% patients with A1C of 7.5% [HR 0.86; 95% CI 0.73“1.01]; 16.9% vs 20.7% patients with A1C >7.5% [HR 0.79; 95% CI 0.66“0.94]; P-interaction 0.47). Finerenone also reduced the incidence of the key secondary CV outcome compared with placebo regardless of baseline A1C (HR 0.88; 95% CI 0.711.07 for A1C ≤7.5% and HR 0.83; 95% CI 0.69“1.01 for A1C >7.5%; P-interaction 0.73). Reduction in UACR was consistent across subgroups. Overall, hyperkalemia events were independent of A1C level. Conclusions: In patients with CKD and T2D, treatment with finerenone reduced the risk for developing adverse kidney and CV outcomes independent of baseline A1C. |
Abstract 14: The Role of Leptin and Inflammatory Related Biomarkers in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Purpose: Leptin is a member of the cytokine family. Its receptor (LEPR-b) is the longest form receptor expressed in cells of the immune system; wherein LEPR-b deficiency causes a decrease in CD4+ cells. LEPR-b is located in hypothalamic and brain stem nuclei, and it primarily regulates energy status. As well, leptin indirectly regulates widespread pain and exercise tolerance by decreasing circulating cortisol. Hyperinsulinemia increases leptin production in adipocytes on a diurnal rhythm; however, the precise relationship between insulin, leptin and pro-inflammatory markers remains uncertain. In clinical settings, high-sensitivity C-reactive protein (hsCRP) has been widely used, as an inflammatory predictor for leptin-related cardiometabolic outcomes and chronic inflammatory symptoms. Leptin-related metabolic and inflammation dysregulations have been clinically reported in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), but not fully elucidated. We examined the association of plasma insulin, leptin, and hsCRP levels with ME/CFS self-reported symptom severity. Methods: Prospective analyses were conducted on ME/CFS patients who met Fukuda/CDC criteria at Birmingham hospital, Alabama, U.S.A. The independent variables were hyperinsulinemia (>174 μIU/ml), hyperleptinemia/hypoleptinemia (>18.3 / <3.3 ng/ml), residual inflammation risk (hsCRP ≥2 and ≤ 26.2 mg/L) and within-individual-variability (WIV) for each biomarker. WIV was defined for each individual as standard deviation/sample residuals adjusting for time and calculated from once-daily random plasma samples over 10-12 weeks. The primary outcomes were: 1) ME/CFS symptom score trends [generalized pain, persistent fatigue, sleep disturbance, impairment of concentration and memory (brain fog), and post exertional malaise (PEM)] calculated from the MFI-20 questionnaire with anchors from 0 to 100 and recorded once daily over a matching 12-14 weeks, and 2) dichotomized symptom severity, with severe symptoms defined as scores > 60/100. After adjusting for age and time, we reported: 1) standard errors (SEM) and p-values for symptom trends using multivariable mixed-effect linear regression models, and 2) odds ratios for severe symptoms using multivariable alternating logistic regression models. Results: We included 29 ME/CFS patients. All were females and >18 years old. Hyperinsulinemia, hyperleptinemia/hypoleptinemia, and residual inflammation risk were 7%, 80%/7%, and 74%, respectively. The medians of insulin-WIV, leptin-WIV and hsCRP-WIV were [(0.24; IQR 0.15-0.38), (0.25; IQR 0.15-0.40), (0.33; IQR 0.18-0.51)] respectively. On average, hyperleptinemic patients had the highest leptin-WIV and 50% of them had residual inflammation risk. Severe (fatigue, pain, brain fog, sleep disturbance, and PEM) were reported in 50%, 29%, 41%, 30%, and 57% of patients, respectively. In the adjusted analysis, worse fatigue scores (7.49; SEM, 2.23; p=.002) were associated with higher insulin-WIV. Hyperleptinemia (OR 1.54; 95% CI 1.13-2.09) compared to hypoleptinemia, and residual inflammation risk (OR 1.65; 95% CI 1.21-2.25) were associated with higher odds of severe fatigue. Worse pain scores (7.17; SEM, 2.30; p=.005) were associated with higher leptin-WIV, and (8.45; SEM, 2.25; p=.0009) higher hsCRP-WIV, and residual inflammation risk (OR 1.75; 95% CI 1.34-2.29) was associated with higher odds of severe pain. Severe brain fog scores (9.20; SEM, 2.44; p=.0008) were associated with higher insulin-WIV, higher leptin-WIV (4.73; SEM, 2.12; p=.03). Residual inflammation risk (OR 1.40; 95% CI 1.16-1.77) was associated with higher odds of severe brain fog. Hyperleptinemia (OR 0.60; 95% CI 0.43-1.19) was associated with lower odds of severe PEM compared to hypoleptinemia, and better sleep quality was associated (6.07; SEM, 1.70; p=.001) with higher insulin-WIV, and (3.37; SEM, 1.47; p=.03) higher leptin-WIV. Conclusions: In patients with ME/CFS, symptoms severity was associated with hyperleptinemia, inflammation and within-individual-variability of these biomarkers. Leptin and hsCRP may be clinically useful in predicting symptom severity. Larger clinical trials are needed to further examine the prediction and causality of these biomarkers in the development of ME/CFS diagnosis. The efficacy and safety of anti-inflammatory therapies may be evaluated in sub-clusters of ME/CFS with metabolic responses and inflammation dysregulations to improve patient-reported symptoms. |

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward